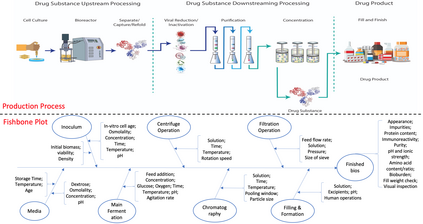
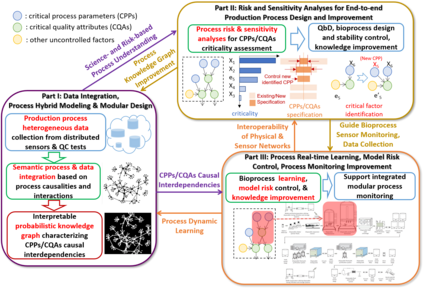
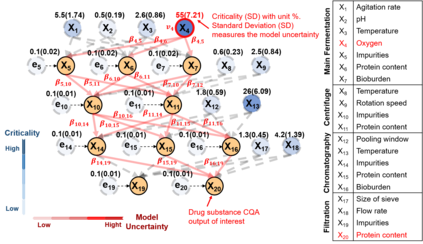
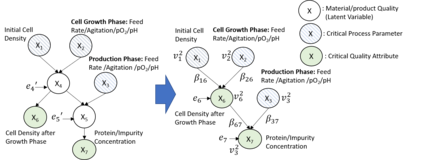
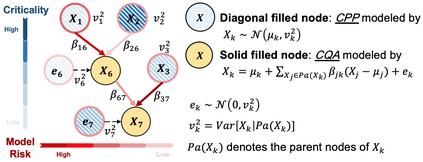
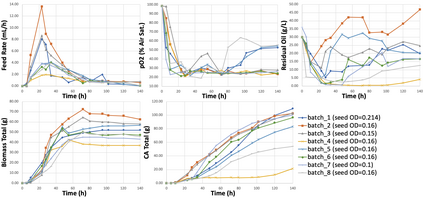
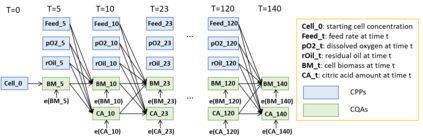
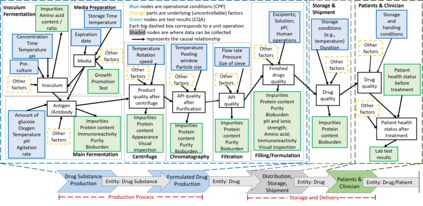
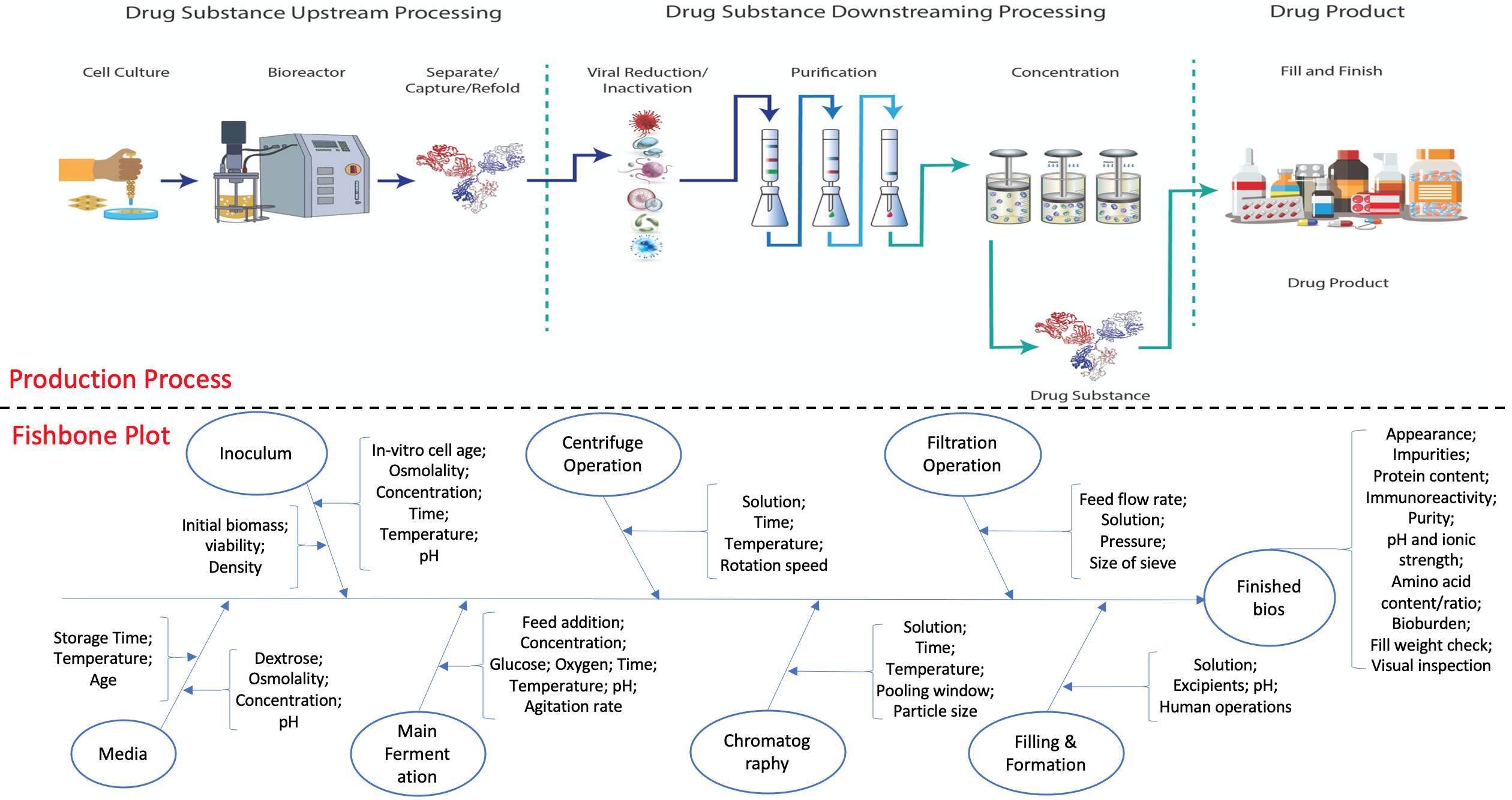
While biomanufacturing plays a significant role in supporting the economy and ensuring public health, it faces critical challenges, including complexity, high variability, lengthy lead time, and very limited process data, especially for personalized new cell and gene biotherapeutics. Driven by these challenges, we propose an interpretable semantic bioprocess probabilistic knowledge graph and develop a game theory based risk and sensitivity analyses for production process to facilitate quality-by-design and stability control. Specifically, by exploring the causal relationships and interactions of critical process parameters and quality attributes (CPPs/CQAs), we create a Bayesian network based probabilistic knowledge graph characterizing the complex causal interdependencies of all factors. Then, we introduce a Shapley value based sensitivity analysis, which can correctly quantify the variation contribution from each input factor on the outputs (i.e., productivity, product quality). Since the bioprocess model coefficients are learned from limited process observations, we derive the Bayesian posterior distribution to quantify model uncertainty and further develop the Shapley value based sensitivity analysis to evaluate the impact of estimation uncertainty from each set of model coefficients. Therefore, the proposed bioprocess risk and sensitivity analyses can identify the bottlenecks, guide the reliable process specifications and the most "informative" data collection, and improve production stability.
翻译:虽然生物制造在支持经济和确保公众健康方面发挥着重要作用,但它面临严峻的挑战,包括复杂性、高变异性、较长的周转时间和非常有限的流程数据,特别是个性化新细胞和基因生物治疗系统。受这些挑战的驱动,我们提出一个可解释的语义生物加工概率学图,并为生产过程开发一个基于游戏理论的风险和敏感性分析,以便利逐个设计和稳定控制质量。具体来说,通过探索关键流程参数和质量属性(CPP/CQAs)的因果关系和相互作用,我们建立了一个基于巴伊西亚网络的概率性知识图,说明所有因素的复杂因果相互依存关系。然后,我们采用了基于敏感性分析的示意值,该图可以准确地量化每种投入因素对产出(即生产率、产品质量)的变异性贡献。由于生物加工模型系数是从有限的流程观测中学习的,我们从巴伊西亚的后传分布中得出模型不确定性和质量属性(CPPs/CQAs),我们创建了一个基于沙普利价值的敏感性分析,以评价各种因果性因素的影响,从每个设定的模型的敏感度分析中确定最可靠的生产复杂性,“稳定性分析” 和生物测定,因此,可以确定最可靠的稳定性系数。